Despite being a diabetes medication, people without the health condition have been buying Ozempic due to its appetite curbing side-effects, which led to global shortages earlier this year. Now, it’s being reviewed by European regulators over a possible connection to thoughts of self-harm among users.
Earlier this year, discourse about weight-loss drugs spread like wildfire online, thought to be influenced by suspected reports of celebrities using them to shed a few pounds.
According to a database maintained by the US Food and Drug Administration, this amounted in an Ozempic shortage worldwide, leaving those truly in need of the medication unable to fill their prescriptions.
The Ozempic injection, which regulates blood sugar levels and insulin for patients with Type 2 diabetes by mimicking a hormone produced in the gut called GLP-1, shot to popularity due to its appetite curbing side-effects.
Now, it’s being reviewed by European regulators over a possible connection to thoughts of suicide and self-harm among users.
According to the BBC, the European Medicines Agency was alerted to the potential link following three cases.
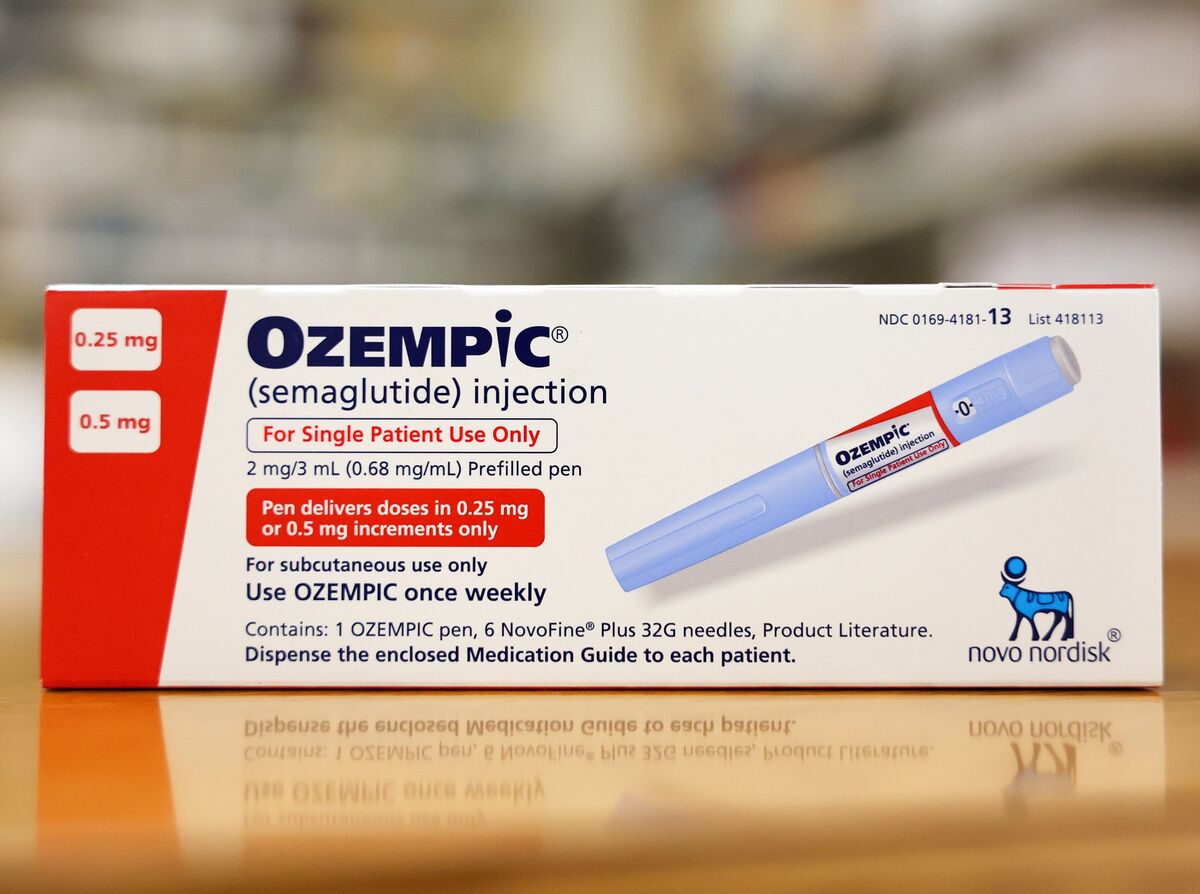
As a result, its Pharmacovigilance Risk Assessment Committee will investigate anything containing either semaglutide or liraglutide.
They will also assess Wegovy, Saxenda and other similar injectable drugs that are comparable to Ozempic, in that they are known to suppress hunger and make you feel full.
‘The review is being carried out in the context of a signal procedure raised by the Icelandic Medicines Agency, following three case reports. A signal is information on a new or known adverse event that is potentially caused by a medicine and that warrants further investigation,’ an EMA official told the BBC.