Oxford University has partnered with pharmaceutical giant AstraZeneca to produce a global coronavirus vaccine and hopefully begin delivering doses by the end of the year.
After preliminary trial results revealed that Oxford University’s Covid-19 vaccine – called AZD1222 – appears to be safe, AstraZeneca has stated that it hopes to begin distributing it by the end of the year.
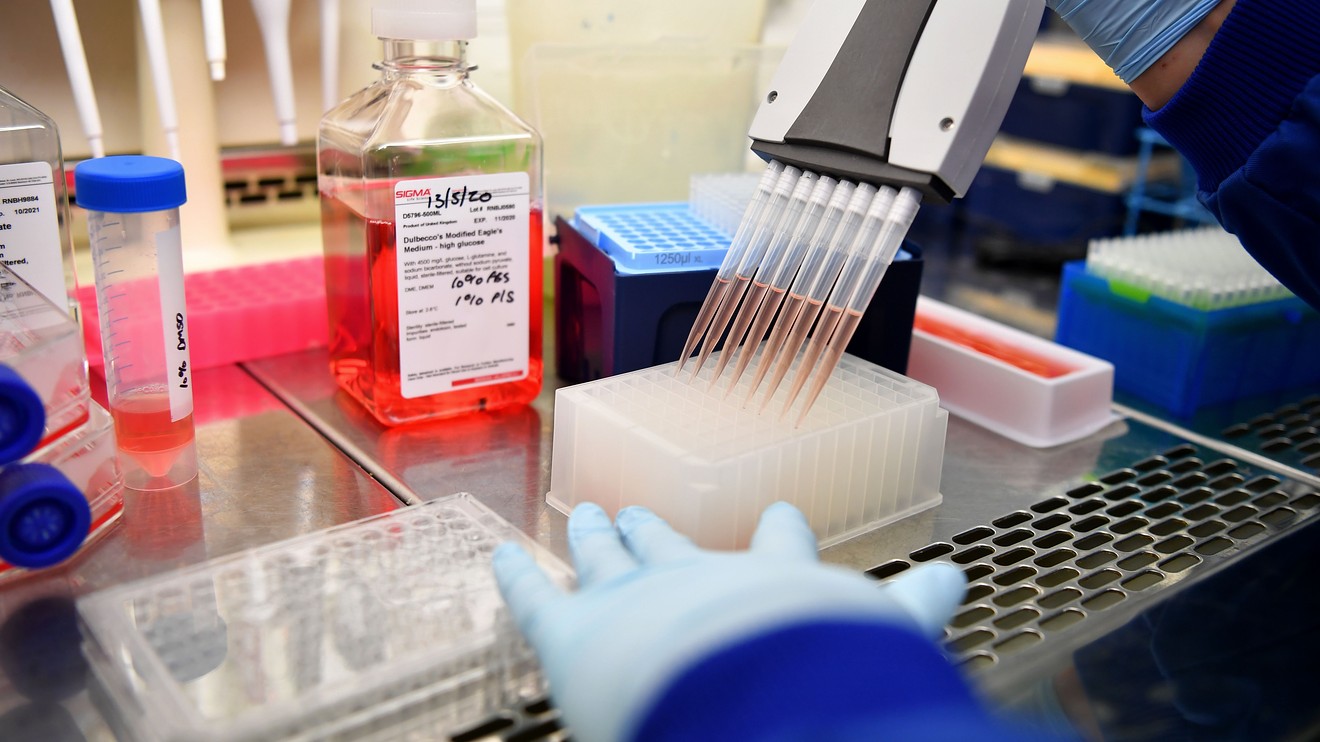
Currently in the early stages of clinical human trials involving 1,000 healthy adult volunteers, AZD1222 has been listed by WHO as the leading candidate in the race to finally bring an end to the crisis that’s already killed more than 600,000 people.
Although over 150 possible vaccines are in various stages of development at the moment, having demonstrated no signs of serious side effects, AZD1222 marks the most promising breakthrough so far.
In data published on Monday by the Lancet journal, scientists have confirmed that the vaccine provokes a dual immune response amongst people aged between 18 and 55, inducing a T-cell response within two weeks and an antibody response after just one month.
Made from a genetically engineered virus that has been modified, the vaccine looks like coronavirus, but has no way of triggering the infection in humans. Instead, it trains the immune system to attack.
JUST IN: Oxford University’s coronavirus vaccine with AstraZeneca shows a positive immune response in an early trial. @MegTirrell has the details. https://t.co/GY9hSS1MTw pic.twitter.com/PkhP4zShAZ
— CNBC (@CNBC) July 20, 2020
What’s yet to be discovered, is how long these cells can survive inside the body and whether or not the vaccine can prevent people from becoming unwell or even experiencing coronavirus symptoms at all.
‘There is still much work to be done before we can confirm if our vaccine will help manage the Covid-19 pandemic,’ says co-author of the study, Professor Sarah Gilbert. ‘But these findings are indeed very positive news. We hope this means the immune system will remember the virus, so that our vaccine will protect people for an extended period.’
Should it prove 100% effective and eventually gain the necessary regulatory approval, AstraZeneca has signed agreements with governments across the globe to supply AZD1222. The pharmaceutical giant has explicitly said it will not be seeking to gain any form of profit while the pandemic persists.
‘We’re on track to be producing doses by September,’ says CEO of AstraZeneca, Pascal Soriot. ‘But we’re hoping it’ll be available this year if late-stage trials can be completed as quickly as we want.’